The Holy Grail for clinicians is reducing the high “residual” cardiovascular risk that persists in high-risk patients despite our best evidence-based treatment including statins. Overwhelming evidence still backs LDL as the primary target for intervention. Yet, beyond statin therapy, to date only the IMPROVE-IT trial has shown modest benefit (by 6.4%) in reducing cardiovascular outcomes by adding non-statin treatment – Ezetimibe, a cholesterol absorption inhibitor – to statin therapy in high cardiovascular risk patients.
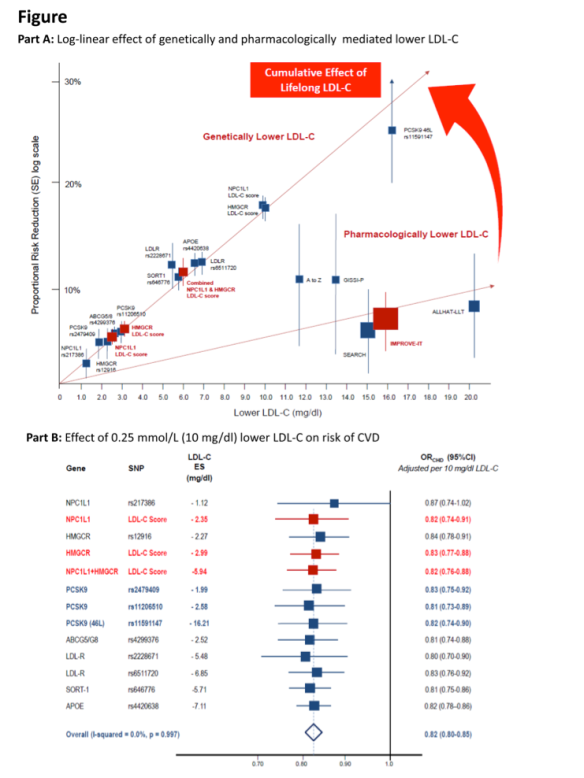
 Hope has focused on PCSK9 inhibition as a therapeutic strategy that may address this unmet clinical need. Indeed, in individuals with genetic variants in PCSK9, lifelong reduction in LDL concentration was associated with up to 88% reduction in coronary events over a 15 year follow-up period.1 The development of monoclonal antibodies to PCSK9 heralds a new era in LDL lowering and cardiovascular disease prevention. The accumulating evidence shows that these PCSK9 inhibitors reduce LDL consistently by 50-60%, across a spectrum of patients and concomitant LDL-lowering therapy including statins. Of great interest these treatments also reduce lipoprotein (a), an established cardiovascular risk factor and potential contributor to residual cardiovascular risk, by 25-30%.2 8
Hope has focused on PCSK9 inhibition as a therapeutic strategy that may address this unmet clinical need. Indeed, in individuals with genetic variants in PCSK9, lifelong reduction in LDL concentration was associated with up to 88% reduction in coronary events over a 15 year follow-up period.1 The development of monoclonal antibodies to PCSK9 heralds a new era in LDL lowering and cardiovascular disease prevention. The accumulating evidence shows that these PCSK9 inhibitors reduce LDL consistently by 50-60%, across a spectrum of patients and concomitant LDL-lowering therapy including statins. Of great interest these treatments also reduce lipoprotein (a), an established cardiovascular risk factor and potential contributor to residual cardiovascular risk, by 25-30%.2 8
Two urgent questions remain. Does this substantial LDL lowering translate to reduction in cardiovascular outcomes in high cardiovascular risk patients?
Well we now have early data to suggest that the promise of PCSK9 inhibition may deliver. At an eagerly anticipated hotline at the American Congress of Cardiology 2015, just a few day ago, a pre-specified, exploratory analysis of the OSLER studies, showed that the PCSK9 monoclonal antibody evolocumab reduced low-density lipoprotein (LDL) by 61%, and that this was associated with a 53% reduction in cardiovascular events over nearly 12 months in high cardiovascular risk patients, including those with familial hypercholesterolaemia (inherited high cholesterol, FH).
The results were consistent with those from a post hoc analysis from the ODYSSEY LONGTERM study with alirocumab, first reported at the European Society of Cardiology Congress, Barcelona, 2014. Both reports were simultaneously published online at The New England Journal of Medicine.
OSLER analysis
This analysis was based on data from 4,465 patients with mean age 58 years, 80% with at least one cardiovascular risk factor including 10% with FH, 70% on statins. Patients were randomly allocated in OSLER-1 and OSLER-2 to open-label treatment with evolocumab (140 mg every 2 weeks or 420 mg every month, n=2976) on top of standard therapy, or standard therapy alone (n=1489).
Efficacy
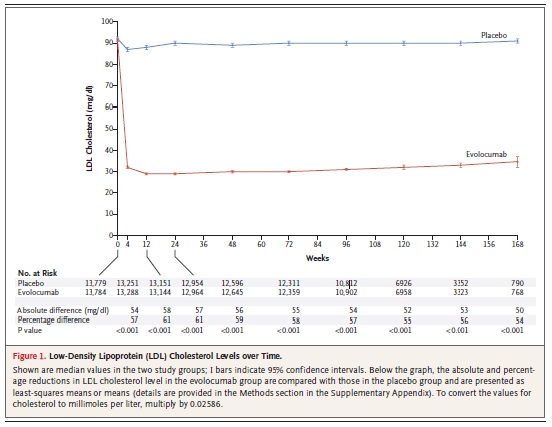
After 12 weeks, evolocumab reduced LDL from 120 mg/dL (3.1 mmol/L) at baseline to a median of 48 mg/dL (1.2 mmol/L), representing a 61% reduction versus standard therapy. At this time, 90.2% of patients attained an LDL target <100 mg/dL (2.6 mmol/L) versus 26.0% on standard therapy alone, and 73.6% attained an LDL target <70 mg/dL (1.8 mmol/l) versus 3.8% on standard therapy alone. Evolocumab treatment also reduced lipoprotein (a) by 25.5%. These lipid changes were generally sustained over the 11.1 month follow-up. In a pre-specified exploratory analysis, treatment with evolocumab reduced cardiovascular events (a composite of death, myocardial infarction, unstable angina requiring hospitalisation, coronary revascularisation, stroke, transient ischaemic attack and heart failure requiring hospitalisation) by 53% (Kaplan Meier estimates at 1 year, 0.95% with evolocumab and 2.18% with standard therapy, Hazard ratio 0.47, 95% CI 0.29-0.78, p=0.003).
Safety
Safety analyses showed that adverse event rates were generally similar between the two groups, (overall event rates 69.2% and 64.8%). There was no evidence of any increase in muscle-related symptoms (6.4% versus 6.0%), liver enzyme elevation > 3 x upper limit of normal (1.0% and 1.2%) and creatine kinase increase > 5 x upper limit of normal (0.6% versus 1.1%). Neurocognitive events, although few, were reported more with evolocumab than standard therapy (27 [0.9%] versus 4 [0.3%]). However, the risk of adverse events, including neurocognitive events did not correlate with the extent of LDL reduction, and was no more prevalent in those subjects who attained LDL levels below 25 mg/dL (0.65 mmol/L). The authors acknowledged a number of limitations to the report, including the open-label design, the heterogeneity of patients, the low event rates, short follow-up and the fact that the study design only allowed patients who had successfully tolerated evolocumab in the parent study to enter OSLER-1 and OSLER-2. Despite these reservations, the authors highlight the potential of evolocumab treatment, in addition to standard therapy, including statin, for reducing cardiovascular outcomes in high cardiovascular risk patients.
ODYSSEY analysis
The analysis from the ODYSSEY LONG TERM trial included 2,341 high cardiovascular patients with mean age 60 years, 18% with inherited high cholesterol (FH) with LDL levels of 70 mg/dL (1.8 mmol/L) or greater, despite maximally tolerated statin therapy, with or without other lipid-lowering therapy. Baseline LDL was 122 mg/dL (3.2 mmol/L). All patients were randomised to double-blind treatment with alirocumab (150 mg every 2 weeks, n=1553) or placebo (n=788), in addition to standard treatment, every 2 weeks for up to 78 weeks. After 24 weeks, there was a 62% reduction in LDL versus placebo, with mean absolute LDL levels 48 mg/dL (1.2 mmol/L) with alirocumab versus 119 mg/dL (3.1 mmol/L) with placebo. Regardless of risk level, 79.3% of patients in the alirocumab group achieved an LDL goal <70 mg/dL (1.8 mmol/L), compared with 8.0% for the placebo group. Consistent LDL reductions with alirocumab were maintained over the 78 weeks of follow-up. Lipoprotein (a) was reduced by 25.6% at 24 weeks. Using the same endpoint as in ODYSSEY OUTCOMES (major adverse cardiovascular events [MACE], defined as coronary heart disease death, non-fatal MI, ischaemic stroke and unstable angina requiring hospitalisation), a post hoc analysis showed a 48% reduction in MACE over the 78 weeks (absolute event rates 1.7% versus 3.3%, Hazard ratio 0.52, 95% CI 0.31 to 0.90, p=0.02). While it is intriguing that this reduction was on top of statin therapy, caution is needed given that these are findings from a post hoc analysis, based on few events over a relatively short duration of follow-up.
Safety
Safety data was broadly balanced between the two groups (overall rates 81.0% versus 82.5% on placebo). Myalgia rates were higher with alirocumab than placebo (5.4% versus 2.9%), although there was no increase in the numbers of patients with liver enzyme elevations, or with creatine kinase increases greater than 3 x upper limit or normal (3.7% versus 4.9%). As seen with the OSLER studies, there was a small excess of neurocognitive disorders (18 [1.2%] on alirocumab versus 4 [0.5%] on placebo), although it should be borne in mind that absolute numbers of events in each group were small. Additionally, these events were self-reported and not evaluated using a specific neurocognitive tool. It is, however, unlikely that this effect is attributable to the LDL lowering, given that in the OSLER analysis, the risk of adverse events, including neurocognitive events did not correlate with the extent of LDL reduction.
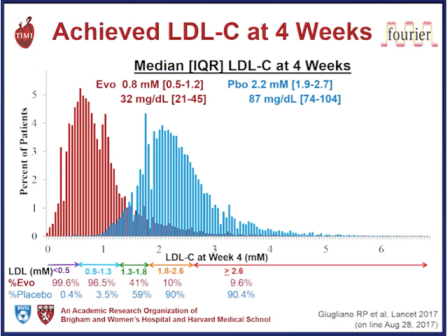
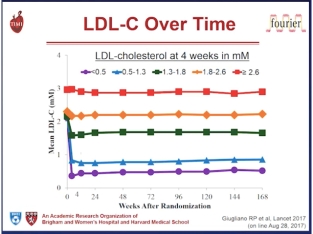
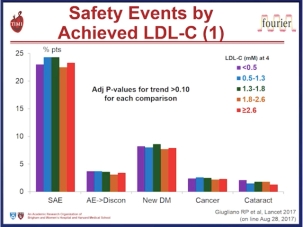
Furthermore, there is no evidence that the presence of loss of function PCSK9 variants is associated with any detrimental effect on neurocognitive function. A dedicated neurocognitive sub study for evolocumab is investigating this issue.3 Clearly, these outcomes and safety data are promising. Ultimately, however, we need to wait for the results of long-term outcomes studies – FOURIER with evolocumab,4 ODYSSEY OUTCOMES with alirocumab,5 and SPIRE-1 and SPIRE-2 with bococizumab,6 7 with these agents to definitively assess the benefit versus risk of PCSK9 inhibition as a therapeutic strategy to reduce residual cardiovascular risk in high risk patients. In addition, as the genetic studies would suggest, in order to eliminate “residual risk” we probably need to treat those at high risk, such as patients with inherited high lipoproteins – familial hypercholesterolaemia (FH) – earlier and more aggressively, in order to delay, or even prevent, the onset of cardiovasular disease.
Watch this space for more on PCSK9 – inhibitors in modifying atherosclerotic vascular disease…….
Blessings
Cardiologydoc
References
| 1. |
|
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354):1264-72. PUBMED abstract: http://www.ncbi.nlm.nih.gov/pubmed/16554528 |
| 2. |
|
Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, Albizem M, Scott R, Stein EA. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol 2014;63:1278-88. PUBMED abstract: http://www.ncbi.nlm.nih.gov/pubmed/24509273 |
| 3. |
|
EBBINGHAUS (Evaluating PCSK9 Binding antiBody Influence oN coGnitive HeAlth in High cardiovascUlar Risk Subjects) [Substudy of FOURIER]. ClinicalTrials.gov Identifier: NCT02207634 |
| 4. |
|
Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk. ClinicalTrials.gov Identifier: NCT01764633 |
| 5. |
|
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab). ClinicalTrials.gov Identifier: NCT01663402 |
| 6. |
|
SPIRE-1 (Evaluation of Bococizumab in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects) ClinicalTrials.gov Identifier: NCT01975376 |
| 7. |
|
SPIRE-2 (Evaluation of Bococizumab in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects). ClinicalTrials.gov Identifier: NCT01975389 |
| 8. |
|
Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, Fisher E, Kovanen PT, Kuivenhoven JA, Lesnik P, Masana L, Reiner Z, Taskinen MR, Tokgözoglu L, Tybjærg-Hansen A; European Atherosclerosis Society Consensus Panel. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 2010;31:2844-53. PUBMED abstract: http://www.ncbi.nlm.nih.gov/pubmed/20965889 |

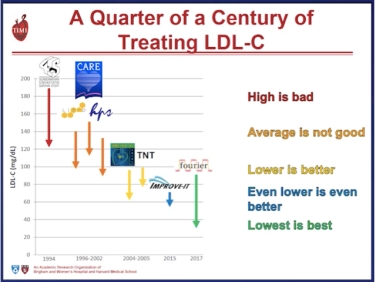
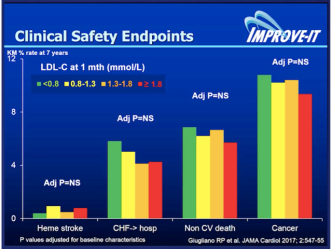
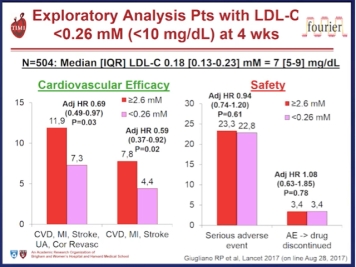
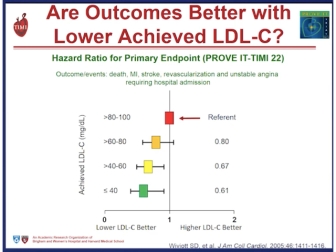 Improved cardiovascular outcome was seen in the Prove IT-TIM 22 trial to achieve very low LDL-C levels < 80 mg/dL (< 2.0 mmol/L) with a 40% risk reduction of events at < 40 mg/dL (1.0 mmol/L). Safety concerns were not an issue despite this aggressive LDL-C lowering.
Improved cardiovascular outcome was seen in the Prove IT-TIM 22 trial to achieve very low LDL-C levels < 80 mg/dL (< 2.0 mmol/L) with a 40% risk reduction of events at < 40 mg/dL (1.0 mmol/L). Safety concerns were not an issue despite this aggressive LDL-C lowering.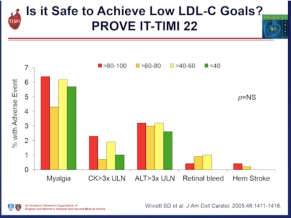
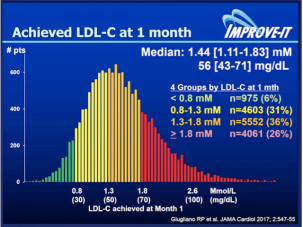
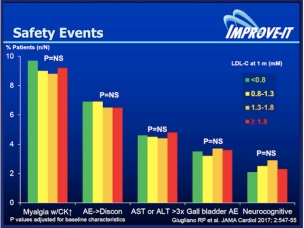
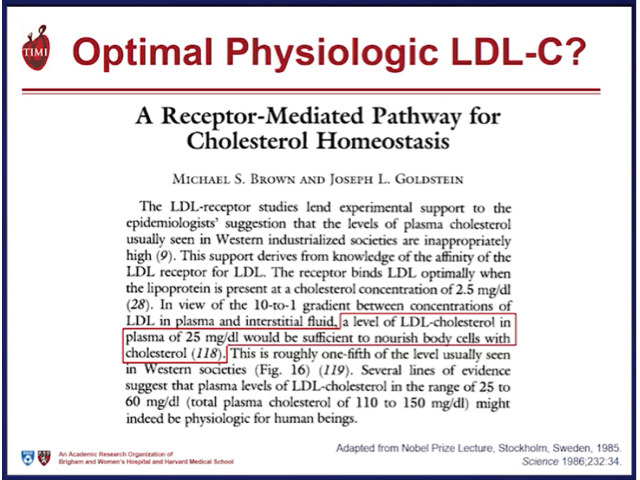

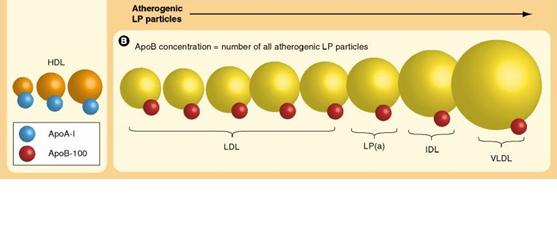
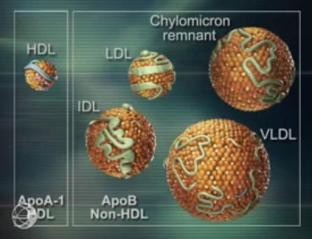 density lipoprotein (LDL) and the other atherogenic lipoproteins (VLDL; IDL and Lipoprotein”a”) the Apo B containing lipoproteins are the cornerstone of the pathophysiology of atherosclerotic vascular disease (ASCVD). The continued debate as well-established modifiable risk factors for cardiovascular disease, is how low to drive these lipoproteins in the prevention and management of ASCVD.
density lipoprotein (LDL) and the other atherogenic lipoproteins (VLDL; IDL and Lipoprotein”a”) the Apo B containing lipoproteins are the cornerstone of the pathophysiology of atherosclerotic vascular disease (ASCVD). The continued debate as well-established modifiable risk factors for cardiovascular disease, is how low to drive these lipoproteins in the prevention and management of ASCVD.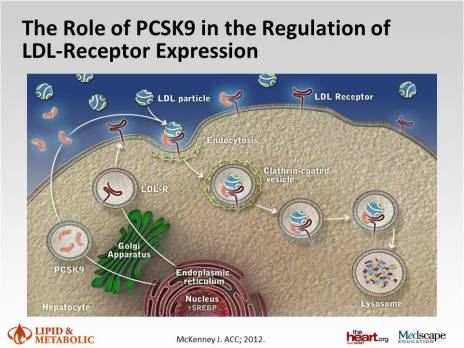


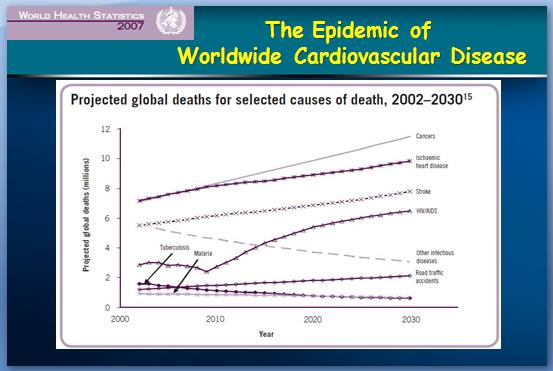
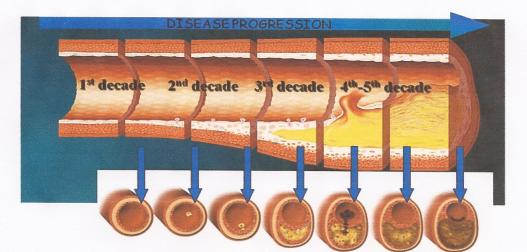
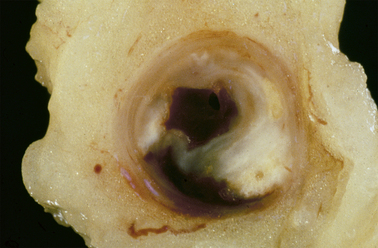
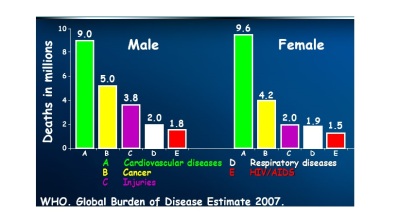 While women do tend to be protected from cardiovascular disease throughout their childbearing years, this protection falls away rapidly after the menopause, when they are more likely to die from cardiovascular disease than from any other illness.
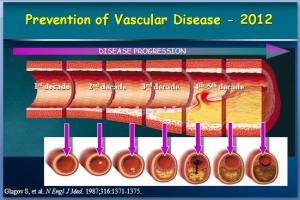
While women do tend to be protected from cardiovascular disease throughout their childbearing years, this protection falls away rapidly after the menopause, when they are more likely to die from cardiovascular disease than from any other illness.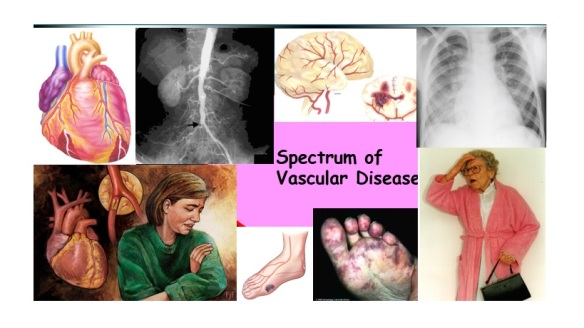 Atherosclerosis of our arteries is a diffuse disease generally affecting many vascular
Atherosclerosis of our arteries is a diffuse disease generally affecting many vascular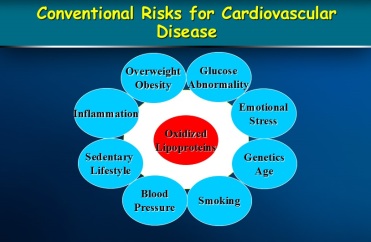 People with established cardiovascular disease require the most intensive lifestyle and medication intervention.
People with established cardiovascular disease require the most intensive lifestyle and medication intervention.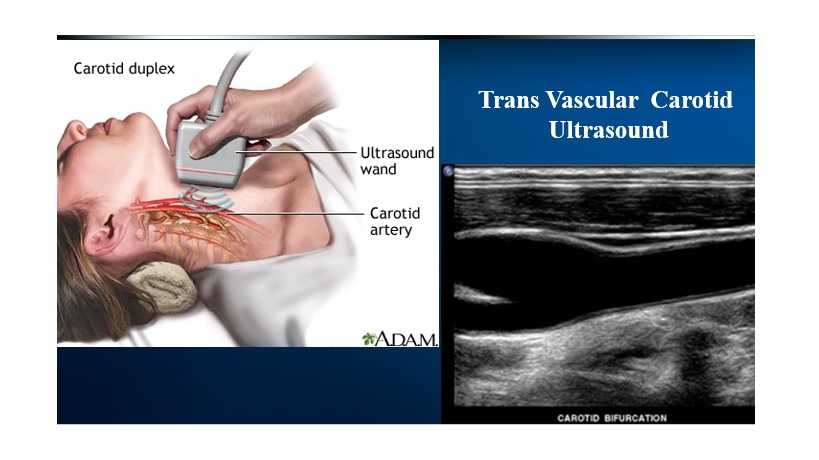




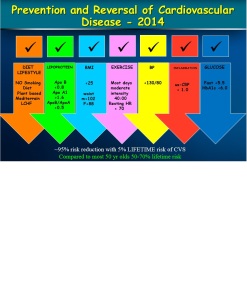
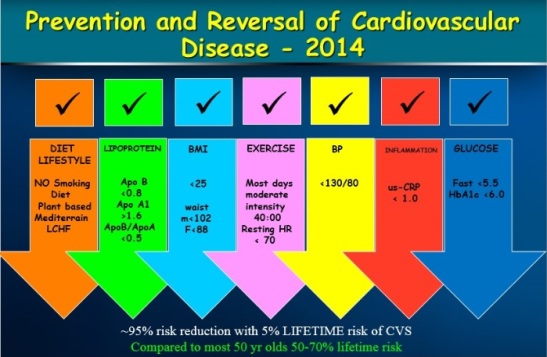
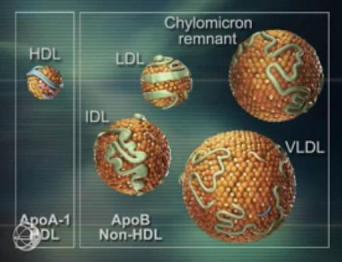 With optimal lifestyle; diet and exercise oxidation and glycation of lipoproteins is reduced leading to lower “systemic” inflammation and lower rates of plaque instability. Apparently healthy individuals, should be assessed for total cardiovascular risk over the short term (10 years) and their lifetime as clinical trials have shown improved survival rates with aggressive Apo B lowering with extremely low Apo B/ Apo A1 ratio. The reduction in morbidity and mortality is through plaque stabilization and plaque reduction.
With optimal lifestyle; diet and exercise oxidation and glycation of lipoproteins is reduced leading to lower “systemic” inflammation and lower rates of plaque instability. Apparently healthy individuals, should be assessed for total cardiovascular risk over the short term (10 years) and their lifetime as clinical trials have shown improved survival rates with aggressive Apo B lowering with extremely low Apo B/ Apo A1 ratio. The reduction in morbidity and mortality is through plaque stabilization and plaque reduction.